 In the previous blog conclusive evidence was presented showing that some vaccines contain residual human fetal cell material from the growth mediums used in vaccine manufacture. Most people are unaware that the shots they inject into babies contain fragments of cells from human babies, and some even deny that this is true.
In the previous blog conclusive evidence was presented showing that some vaccines contain residual human fetal cell material from the growth mediums used in vaccine manufacture. Most people are unaware that the shots they inject into babies contain fragments of cells from human babies, and some even deny that this is true.
Four significant vaccines on the CDC recommended schedule [1] all contain human fetal tissue fragments, including both male and female DNA:
- M-M-R®II: exclusively available in the USA since 1979 targeting measles, mumps, and rubella; 2 doses at 12 months and 5 years. [2]
- Varivax®: the vaccine aimed to prevent chicken pox, added to the schedule in 1996; 2 doses at 12 months and 5 years.
- Hepatitis A vaccine, approved in 1996, and added to the schedule for all babies in 2005; 2 doses given between 12 and 24 months. [3]
- Pentacel® combined vaccine targeting diphtheria, tetanus, acellular pertussis, polio, Haemophilus influenza type b (Hib), introduced in 2008. Four doses given at 2, 4, 6, and 15 months of age. [4]
The average baby is injected with 10 different human tissue-containing shots before they go to school. Eight of them before the age of two years. Surely such widespread use of this growth medium – human fetal cells – was thoroughly demonstrated to be safe – right?
 Since 40 years have passed since the first vaccine containing human cell debris was introduced, there has been ample time to study how this vaccine containing human DNA fragments could be impacting those that are injected with it.
Since 40 years have passed since the first vaccine containing human cell debris was introduced, there has been ample time to study how this vaccine containing human DNA fragments could be impacting those that are injected with it.
But, how much DNA is really in a vaccine? Isn’t it just infinitesimally small amounts?
DNA residuals in human fetal cell line manufactured vaccines
In addition to the ingredients listed on the package insert for Meruvax II® (rubella), we detected significant levels of human ssDNA (142 ± 8 ng/vial) as well as dsDNA (35 ±10 ng/vial) fragmented to ~215 base pairs in length. The MMR II® package insert discloses the presence of human fetal residuals [sic] [but not] how much cell substrate dsDNA or ssDNA contaminates each dose. In each vial of Havrix® [Hepatitis A vaccine], we detected ssDNA (301 ± 153 ng/vial) as well as dsDNA (44 ± 24 ng/vial) unfragmented residual DNA more than 48.5 K base pairs in length. The Havrix® package insert discloses the presence of human fetal cellular residuals from the MRC-5 cell line, but not the DNA contaminant levels specifically.[5]
The Varivax® vaccine [chicken pox] is manufactured using the human diploid cell line MRC5, and is contaminated with 2 micrograms of cell substrate double stranded DNA. Single stranded DNA levels are not reported in Merck’s Varivax Summary Basis for Approval document nor are the length of the DNA fragments contaminating the vaccine (Merck 2011). [5]
Vaccines that have been cultured on or manufactured using the WI-38 fetal cell line such as MeruvaxII®, MMRII®, Varivax®, Havrix® and Pentacel® are additionally contaminated with fragments of human endogenous retrovirus HERVK (Victoria et al., 2010). Recent evidence has shown that human endogenous retroviral transcripts are elevated in the brains of patients with schizophrenia or bipolar disorder (Frank et al., 2005), [5]
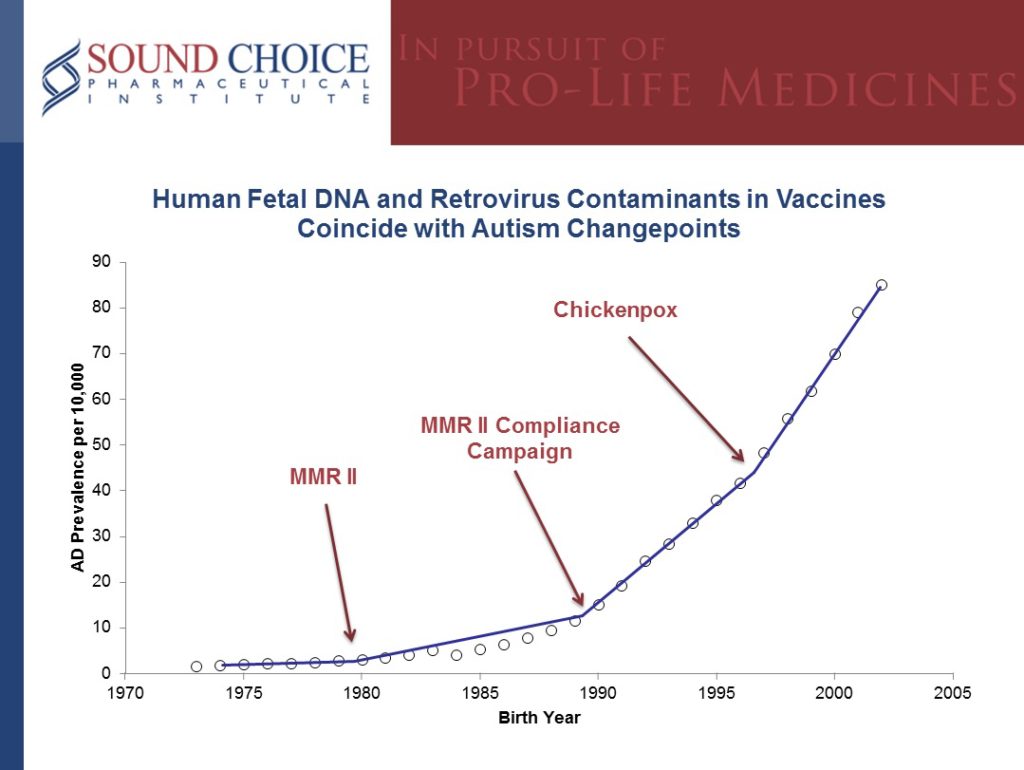
According to EPA recommendations, birth year change points for prevalence of autistic disorder should drive consideration of environmental triggers, as for any disease (McDonald 2010).[5]
Scientists have been studying and learning that injected “human fetal DNA fragments are inducers of autoimmune reactions, while both DNA fragments and retroviruses are known to potentiate genomic insertions and mutations (Yolken et al., 2000; Kurth 1998; U S Food and Drug Administration 2011).” [5]
How has injecting male and female DNA fragments into ALL babies impacted their health?
A detailed analysis of the data available and has found startling results. There are statistically obvious change points associated with the addition of fetal cell line vaccines and increased diagnosis of autism spectrum disorder: “Autistic disorder began to rise in the US after birth year 1978 (Newschaffer and Gurney 2005).” This corresponds to the introduction of the MMRII developed with two different fetal cell tissues. [5]
Additionally, “The US 1988.4 change point corresponded to the addition of a second dose of MMRII® to a measles vaccination campaign that increased compliance from ≤50 to 82% between birth years 1987 and 1989 (Centers for Disease Control 1989; Kaye and Jick 2001) as well as to the introduction of Poliovax in 1987. [5]
And, “The 1995.6 autistic disorder change point corresponded to the approval and introduction of the Varicella vaccine (Varivax®).” [5]
This chart summarizes the autism change points in relationship to the MMRII, the push for higher uptake of MMRII, and the chicken pox vaccine. [5]
 Why aren’t the FDA (Food and Drug Administration), HHS (Federal Department of Health and Human Services), the CDC (Federal Center for Disease Control), or the ACIP (Advisory Committee on Immunization Practices) concerned about fetal cell contamination in vaccines causing harm?
Why aren’t the FDA (Food and Drug Administration), HHS (Federal Department of Health and Human Services), the CDC (Federal Center for Disease Control), or the ACIP (Advisory Committee on Immunization Practices) concerned about fetal cell contamination in vaccines causing harm?
 The primary measure of effectiveness for the CDC, FDA, and vaccine makers is focused on “serologic evidence of immunity,” or a blood test showing raised antibody titers. No vaccine has ever been investigated for mutagenic or carcinogenic properties – tested and tracked long-term to see if they damage the genetic material of the recipient, if they could be implicated in causing cancer, or if they could be linked to infertility later in life. [6]
The primary measure of effectiveness for the CDC, FDA, and vaccine makers is focused on “serologic evidence of immunity,” or a blood test showing raised antibody titers. No vaccine has ever been investigated for mutagenic or carcinogenic properties – tested and tracked long-term to see if they damage the genetic material of the recipient, if they could be implicated in causing cancer, or if they could be linked to infertility later in life. [6]
Even with all the advances in genetic understanding since the mapping of the human genome in 2001, the HHS has undertaken NO FURTHER SAFETY STUDIES on these vaccines known to contain human fetal DNA fragments. Further, the HHS has done no safety studies at all on any vaccine for 30+ years.
Isn’t that interesting.
You might be asking, ‘But aren’t the vaccine manufacturers responsible for determining safety?’ Ever since the 1986 National Childhood Vaccine Injury Act, all liability was removed from vaccine manufacturers and the responsibility for vaccine safety was shifted to the HHS, who recently admitted, after being forced by a court order, that no safety testing of vaccines has been undertaken. [7]
In June 2018 I had three minutes during the public comment session at the ACIP meeting held three times a year at the CDC in Atlanta. I briefly presented some of the unintended consequences of the vaccine schedule, commonly known as “non specific effects.” It remains to be seen if this information will drive any change in recommendations.
The vaccine promoters have captured the media through controlling advertising revenue. Fear campaigns are promoted so that parents rush to stay up-to-date on vaccines without examining the ingredients. Doctors are busy and have confidence in the government regulatory agency recommendations. Has our cherished vaccine program helped children avoid short term infectious illness but caused an epidemic in longterm serious developmental impairment and auto-immune disorders?
If you have any fear of your child getting chicken pox, please read the description provided by the CDC: “The clinical course in healthy children is generally mild, with malaise, pruritus (itching), and temperature up to 102°F for 2 to 3 days.” [8]
 Would you rather your child have a mild fever and have some itching, or inject them with human cellular debris containing DNA fragments – which has not been tested for whether or not it may adversely impact genetics, play a role in skyrocketing childhood cancers, or impact your future ability to have grandchildren?
Would you rather your child have a mild fever and have some itching, or inject them with human cellular debris containing DNA fragments – which has not been tested for whether or not it may adversely impact genetics, play a role in skyrocketing childhood cancers, or impact your future ability to have grandchildren?
So, today the public is pushed to continue to inject their babies with both male and female DNA, with no investigation of the possible mutagenic (genetic alteration) impact it might be having. We watch sky-rocketing rates of childhood cancer and donate money to those searching for ‘cures’. Many parents watch helplessly as their adult children struggle with infertility, but very few make any connection to vaccines. Vaccines were never studied to impact any of that.
Does this seem like “safe” science to you?
Please share this information widely.
I highly recommend that you read the full paper by Theresa Deisher on Impact of Environmental Factors on the Prevalence of Autistic Disorder after 1979 published in the Journal of Public Health and Epidemiology on 9 July 2014.
 Author: Becky Hastings, wife, mother, grandmother, passionate follower of Jesus and truth. As a breastfeeding counselor for over 25 years, Becky is devoted to helping parents make wise decisions for the long-term health and wellbeing of their babies. As a member of a Vaccine Safety Education Coalition, Becky writes and speaks on the topic of vaccine safety. Becky also loves mountain biking and appreciates all comments and the rare donation which provides wonderful encouragement.
Author: Becky Hastings, wife, mother, grandmother, passionate follower of Jesus and truth. As a breastfeeding counselor for over 25 years, Becky is devoted to helping parents make wise decisions for the long-term health and wellbeing of their babies. As a member of a Vaccine Safety Education Coalition, Becky writes and speaks on the topic of vaccine safety. Becky also loves mountain biking and appreciates all comments and the rare donation which provides wonderful encouragement.
[1] The 2018 (current) CDC vaccine schedule: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#f3
[2] Complete vaccine package insert for the M-M-R®II, exclusively used in the USA since 1979. https://www.fda.gov/downloads/BiologicsBloodVaccines/UCM123789.pdf
[3] Hepatitis Vaccine is manufactured by both Merck and GlaxoSmithKline. Havrix® by GSK was approved for use in the US in 1995; Vaqta® by Merck was approved in 1996. However, Hepatitis vaccine was for limited population groups and not part of the childhood immunization schedule nor recommended for use by any states. In 1999, 17 states began recommending/considering its use for children 24 months and older, and in 2005 it was included in the ACIP recommended vaccination schedule for children 12 months and older. http://soundchoice.org/scpiJournalPubHealthEpidem092014.pdf
“To produce each vaccine, cell culture-adapted virus is propagated in human fibroblasts, purified from cell lysates, inactivated with formalin, and adsorbed to an aluminum hydroxide adjuvant.” The GSK version also has a preservative, 2-phenoxyethanol. https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html
[4] Vaccine package insert for Pentacel combination vaccine https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm109810.pdf
[5] Deisher, Theresa A, et al. “Impact of Environmental Factors on the Prevalence of Autistic Disorder after 1979.” Sound Choice Pharmaceuticals, Journal of Public Health and Epidemiology, 9 July 2014, http://soundchoice.org/scpiJournalPubHealthEpidem092014.pdf.
[6] https://www.cdc.gov/vaccines/pubs/pinkbook/rubella.html
[7] https://www.icandecide.org/health-and-human-services/
[8] https://www.cdc.gov/vaccines/pubs/pinkbook/varicella.html
